Following Anaerobic Digestion of Pretreated Algae by Calorimetry
Michael A.A. Mathews, Ph.D. and Lee D. Hansen, Ph.D.
TA Instruments, 890 West 410 North, Lindon, UT 84042, USA
Introduction
Conversion of renewable biomass to a useable and sustainable fuel is an important part of the move to reducing carbon footprint and reliance upon foreign oil. Algae are able to rapidly sequester CO2 from the combustion of fossil fuels as well as being able to effectively remove phosphate from sewage effluent. Using algal biomass to make biogas is thus a win/win scenario as both the production of the algae and the further downstream use of the algal biomass both have positive environmental impacts.
The algae must be pretreated to maximize digestion by anaerobic bacteria. This digestion produces biogas and is expected to destroy any algal toxins present. The waste from anaerobic digestion is rich in nitrogen and phosphorus and can be used for fertilizer. The waste from production of biodiesel from algae can also be used as feedstock for anaerobic digestion. Anaerobic digestion involves exothermic reactions, and the rates and total heat from these reactions is readily measured by calorimetry. Here, a TAM Air 8-channel calorimeter is used for rapid and real-time measurement of the efficiency of the digestion and therefore of the efficacy of the pretreatments.
Experimental
The bacterial inoculum for the experiments was grown in a 10L anaerobic digester, initially inoculated with manure from a dairy farm. The resulting mixed culture of bacteria includes Clostridium, Flavobacterium, Bacteroides, Spirochaeta, Methanobrevibacter, and Methanosarcina as well as several minor species.
Samples of wet algae were obtained from sewage treatment ponds in Cache County, Utah. The pretreatments tested involved adding various amounts of hydrogen peroxide (30% H2O2 in ratios of 5 mL, 3 mL, or 0.5 mL per gram) and exposing the mixture to UV radiation (a 30 W deuterium lamp focused on approximately 7 cm2 with stirring and irradiation times varied from 0.5 to 8 hours) to destroy any excess peroxide. The samples were mixed with the peroxide, diluted with water to a constant volume, and then exposed to the UV light. The samples were then dried and ground with a mortar and pestle. 1g samples of each were placed in a 10 mL vial and 0.3g of anaerobic bacterial culture was added along with 7 mL of water. The vials were flame sealed, equilibrated at 37°C for 1 hour, and then placed in the TAM Air instrument for 4 days of heat production data collection.
Results and Discussion
If we calculate total heat for each experiment by integrating the thermograms and normalizing for the amount of sample in each vial, then we get a number that is proportional to the overall conversion efficiency for gas production (see Figure 1). This shows us what the data would look like if we were simply going to evaluate each pretreatment using overall gas production as a guide. We can see from this figure that the most metabolic heat, and therefore the most gas, is produced from samples treated with 3 mL of peroxide. Treatment with 5 mL of peroxide produces less overall heat while treatment with 0.5 mL of peroxide is not significantly different than the control. Irradiating for longer than 0.5 h did not significantly increase heat production.
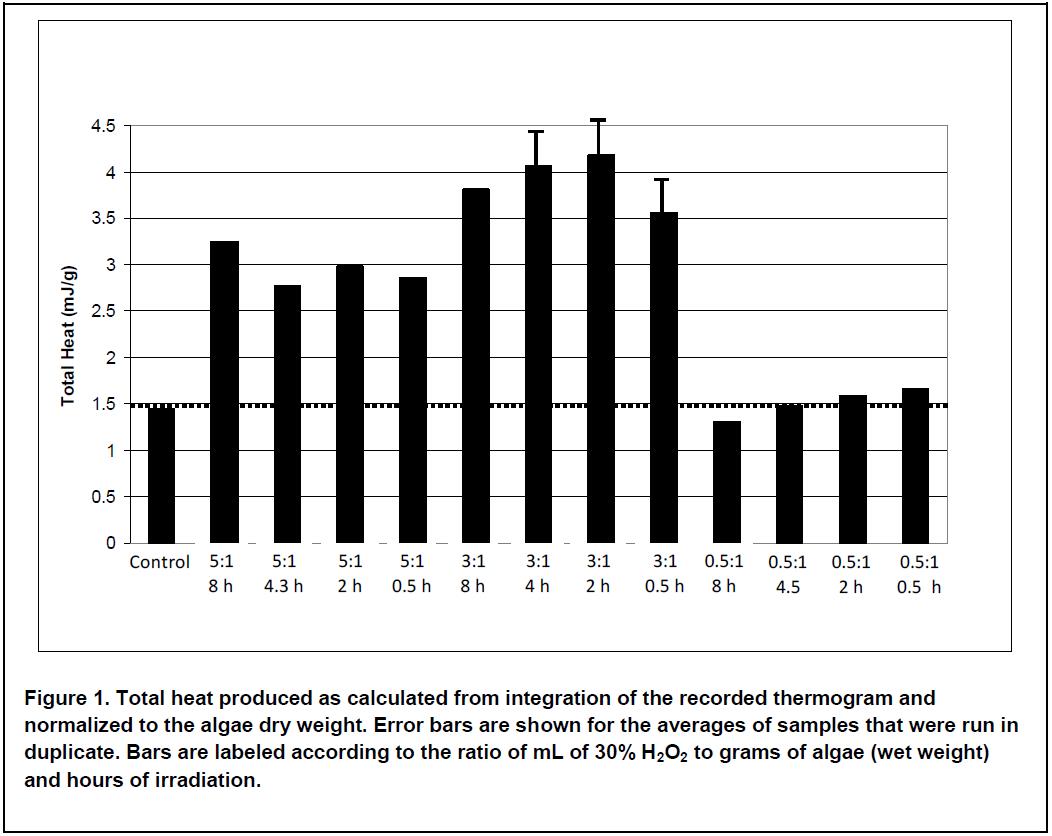
Having used the TAM Air, we can now look at the entire data stream and gain valuable insight into not only the final result, but the details of how each pretreatment affected the anaerobic digestion process.
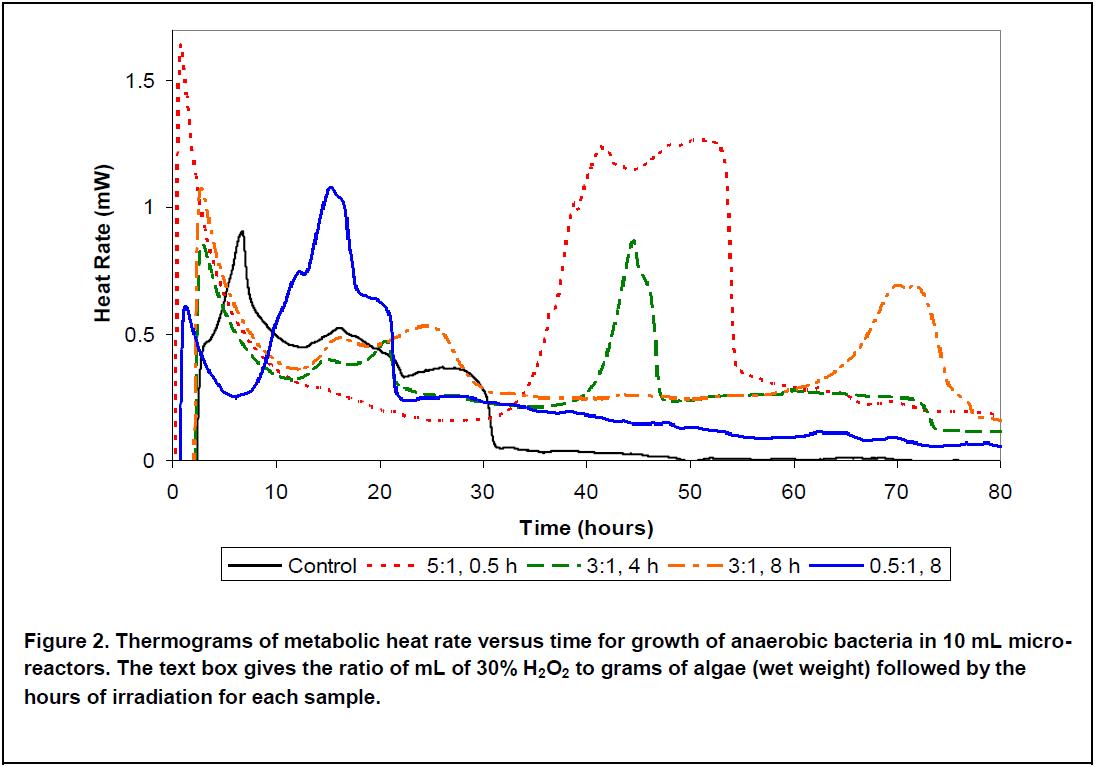
Above (Figure 2), we see several of the thermograms. The control has a major peak with a maximum at 7 hours, this represents the exponential growth of the bacteria. The smaller, broader peaks at 16 and 27 hours are characteristic of intermediate metabolism in mixed cultures. By 31 hours, the metabolic rate has reduced to zero, due to acid production having exceeded the buffer capacity. The other experiments show different peaks, with a variety of residence times before the peak arises. The sample with the lowest amount of peroxide and the longest irradiation time (0.5:1, 8 h) shows a group of peaks centered around 16 hours, with most of the metabolism finishing after about 21 hours, but with a slowly tapering heat proceeding for the rest of the experiment. The largest peak occurs between 33 and 55 hours and is from the highest concentration of peroxide (5 mL per gram) and the shortest irradiation time (0.5 hours).
These thermograms can be used to optimize the pretreatment for the desired residence time and conversion efficiency. Treatment with low peroxide and long irradiation time gives a short residency and reasonable efficiency for gas production. Maximum efficiency of gas production is obtained with higher peroxide concentration and shorter irradiation time, but requires a longer residence time. By showing the time course of the growth and metabolism of the bacteria, these results also provide data that can be used to optimize digester residence time and gas production, thus balancing gas and compost fertilizer production to optimize economic return on investment.
Conclusions
“Calorimetric data were collected and used to measure the effectiveness of various pretreatment methods for digestion of algae by a mixed culture commonly found in anaerobic digesters adapted to cow manure. The method allows rapid and quantitative evaluation of various pretreatments with a minimal amount of materials and sample preparation.” (6)
References
1. Cheong, D.-Y., 2005. Studies of high rate anaerobic bioconversion technology for energy production during treatment of high strength organic wastewaters. Ph.D. Dissertation, Utah State University, Logan, UT.
2. Cline, T., Thomas, N., Shumway, L., Yeung, I., Hansen, C.L., Hansen, L.D., Hansen, J.C., 2010. Method for evaluating anaerobic digester performance. Bioresource Technology, Nov; 101(22): 8623-6..
3. Dustin, J.S., 2010. Fundamentals of operation of the induced bed reactor (IBR) anaerobic digester. Ph.D. Dissertation, Utah State University, Logan, UT.
4. Hansen, C.L.; Hansen, C.S., 2005. Induced sludge bed anaerobic reactor. Patent # 6,911,149 B2, 28 June.
5. Hansen, J.C., Hansen, L.D., 2009. Spiral fin heat-exchanger apparatus and system for heat management. Provisional patent filed 21 August.
6. Mayo, M.H., Nicholson, A.D., Hansen, L.D., Hansen, J.C., 2011. Chemical treatment of algae to facilitate biogas production by anaerobic digestion. Transactions of the ASABE, Vol. 54(4):1-4
7. Wyman, C.E., 2007. What is (and is not) vital to advancing cellulosic ethanol. TRENDS in Biotechnology, 25, 153.
