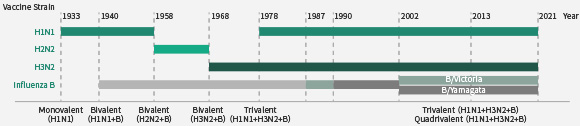
接种流感疫苗是预防流行性感冒的有效方法。每年WHO都会根据新分离出的毒株检测数据以及上一个季节流感疫苗的性能选择3-4种不同的流感毒株作为疫苗株。近几十年,流感疫苗均为4价或者3价,可预防的流感包括1种H1N1、1种H3N2以及1种或者2种B型流感病毒(Yamagata 或/和 Victoria )。
义翘神州ProVir病毒抗原库中覆盖了近600+流感病毒抗原,包括重组HA, NA, NP以及更多流感抗原,覆盖了近些年WHO推荐的所有流感疫苗株,可用于检测分析疫苗介导的抗体应答反应。除此之外,我们还开发了80余种流感病毒单克隆抗体试剂,可用于相关检测试剂开发。
2021-2022流感疫苗株相关重组蛋白产品
| A/Victoria/2570/2019 (H1N1) Recommended for Egg-based (quadrivalent,trivalent) Vaccine HA: 40787-V08H, 40787-V08H1 NA: 40785-V08B NP: 40774-V08B |
A/Wisconsin/588/2019 (H1N1) Recommended for Cell- or recombinant-based (quadrivalent, trivalent) Vaccine HA: 40787-V08H, 40787-V08H1 NA: 40785-V08B NP: 40774-V08B |
| B/Washington/02/2019 Recommended for Egg-based, Cell- or recombinant-based (quadrivalent, trivalent) Vaccine HA: 40722-V08H NA: 40790-V08B NP: 40755-V08B |
B/Phuket/3073/2013 Recommended for Egg-based, Cell- or recombinant-based (quadrivalent) Vaccine HA: 40498-V08B NA: 40502-V07B NP: 40500-V08B |
| A/Cambodia/E0826360/2020 (H3N2) Recommended for Egg-based, Cell- or recombinant-based (quadrivalent, trivalent) Vaccine HA: 40789-V08H, 40789-V08H1 NA: 40784-V08B NP: 40778-V08B (Under Development) |
| A/Guangdong-Maonan/SWL1536/2019 (H1N1) Recommended for Egg-based (quadrivalent, trivalent) Vaccine HA: 40717-V08H NP: 40723-V08B |
A/Hong Kong/2671/2019 (H3N2) Recommended for Egg-based (quadrivalent, trivalent) Vaccine HA: 40721-V08H NP: 40753-V08B |
| A/Hawaii/70/2019 (H1N1) Recommended for Cell- or recombinant-based (quadrivalent, trivalent) Vaccine HA: 40717-V08H NP: 40724-V08B |
A/Hong Kong/45/2019 (H3N2) Recommended for Cell- or recombinant-based (quadrivalent, trivalent) Vaccine HA:40765-V08H (Under Development) NP: 40754-V08B |
| B/Phuket/3073/2013 Recommended for Egg-based, Cell- or recombinant-based (quadrivalent) Vaccine HA: 40498-V08B NA: 40502-V07B NP: 40500-V08B |
B/Washington/02/2019 Recommended for Egg-based, Cell- or recombinant-based (quadrivalent, trivalent) Vaccine HA: 40722-V08H NP: 40755-V08B |
| A/Brisbane/02/2018 (H1N1) Recommended for Egg-based (quadrivalent) Vaccine HA: 40719-V08H NP: 40776-V08B |
A/Kansas/14/2017 (H3N2) Recommended for Egg-based (quadrivalent) Vaccine HA: 40720-V08H NP: 40779-V08B |
| B/Phuket/3073/2013 Recommended for Egg-based (quadrivalent) Vaccine HA: 40498-V08B NA: 40502-V07B NP: 40500-V08B |
B/Colorado/06/2017 Recommended for Egg-based (quadrivalent); trivalent Vaccine HA: 40581-V08H NP: 40782-V08B (Under Development) |
| A/Michigan/45/2015 (H1N1) Recommended for quadrivalent Vaccine HA: 40567-V08H1 NA: 40568-V08B NP: 40777-V08B |
A/Singapore/INFIMH-16-0019/2016 (H3N2) Recommended for quadrivalent Vaccine HA: 40580-V08H NP: 40779-V08B |
| B/Colorado/06/2017 Recommended for quadrivalent; trivalent Vaccine HA: 40581-V08H NP: 40782-V08B (Under Development) |
B/Phuket/3073/2013 Recommended for quadrivalent Vaccine HA: 40498-V08B NA: 40502-V07B NP: 40500-V08B |
| A/Michigan/45/2015 (H1N1) Recommended for trivalent Vaccine HA: 40567-V08H1 NA: 40568-V08B NP: 40777-V08B |
A/Hong Kong/4801/2014 (H3N2) Recommended for trivalent Vaccine HA: 40555-V08B NA: 40569-V08B NP: 40781-V08B |
| B/Brisbane/60/2008 Recommended for trivalent Vaccine HA: 40016-V08B NA: 40203-VNAHC NP: 40783-V08B (Under Development) |
B/Phuket/3073/2013 Recommended for quadrivalent Vaccine HA: 40498-V08B NA: 40502-V07B NP: 40500-V08B |
| A/California/7/2009 (H1N1) Recommended for trivalent Vaccine HA: 11085-V08B NP: 40205-V08B |
A/Hong Kong/4801/2014 (H3N2) Recommended for trivalent Vaccine HA: 40555-V08B NA: 40569-V08B NP: 40781-V08B |
| B/Brisbane/60/2008 Recommended for trivalent Vaccine HA: 40016-V08B NA: 40203-VNAHC NP: 40783-V08B (Under Development) |
B/Phuket/3073/2013 Recommended for trivalent Vaccine HA: 40498-V08B NA: 40502-V07B NP: 40500-V08B |
| A/California/7/2009 (H1N1) Recommended for trivalent Vaccine HA: 11085-V08B NP: 40205-V08B |
A/Switzerland/9715293/2013 (H3N2) Recommended for trivalent Vaccine HA: 40497-V08B NP: 40499-V08B |
| B/Brisbane/60/2008 Recommended for quadrivalent Vaccine HA: 40016-V08B NA: 40203-VNAHC NP: 40783-V08B (Under Development) |
B/Phuket/3073/2013 Recommended for trivalent Vaccine HA: 40498-V08B NA: 40502-V07B NP: 40500-V08B |
